-
 Summary
Summary
Tritium (T) is a radioactive isotope in the hydrogen group, and the almost all of the T in water (H2O) exists as an isotopic water form: HTO. Currently, over 400 facilities of atomic plants and nuclear fuel reprocessing plants in the world are draining radioactive effluents containing T to the ocean and river, without removing.
Recently, we found a suitable oxide-absorbent for extracting T from water at room temperature (http://dx.doi.org/10.1080/01496395.2015.1018440). This novel function that employs an abundant oxide will promote useful developments in the fields of water purification technologies for food, water, and medicine manufacturing industries, nuclear power industry, and advanced bio-industry, etc. However, additional research is needed for treating actual pollutions.
We start this campaign to request supports from society, and to make collaborations with potential organizations in the world, for preventing the large scale water pollution and especially maintaining safe qualities of food & water.

-
 Background
Background
One of the most dreadful catastrophe for modern society is water pollution with radioactive poisons, which increases gene abnormalities and cancers for the all of living things on the earth.
Conventional separation methods (e.g., chemical precipitation or ion-exchange) can remove radioactive nuclides such as 137Cs, 90Sr, 144Ce, 106Ru, and 95Zr from radioactive effluents produced at atomic power plants. However, there are no effective conventional methods for separating tritium (T) forming a water molecule (HTO) from huge volume of water (H2O) at room temperature due to the similarity of physicochemical properties between HTO and H2O. Hence, radioactive effluents containing T are drained to the ocean and river from over 400 of atomic power plants and nuclear fuel reprocessing plants in the world, without removing T from the effluents.
Moreover, the post-accident Fukushima atomic power plant (PAFAP) is currently producing T-containing effluents that have radioactive concentrations of 1,000,000 ~ 5,000,000Bq/L. The EU standard value of T for drinking water is 100 Bq/L. Since the accident, over 900 trillion Bq of T produced from the accident reactors is being stored in tanks by April 2015. This quantity of T in the tanks is equivalent to the quantity of T from the normal plant operation for 450 years. The production of effluent from the PAFAP has been forecast to continue for at least 20-30 years, until the finish of the operation of collecting meltdown fuels.
-
 What is the risk of tritium ?
What is the risk of tritium ?
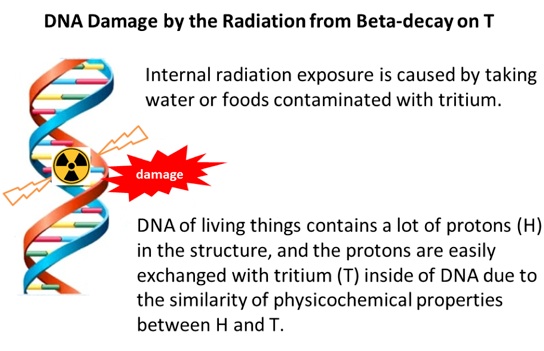
Thus, we must minimize T contamination for maintaining safety of drinking water or food, by removing T from effluents at atomic power plants, before releasing the effluents to the river or ocean.
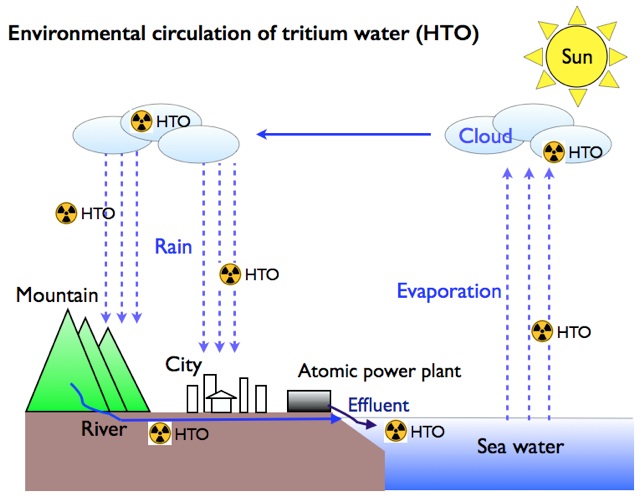
-
 What is the aim of funding on this campaign ?
What is the aim of funding on this campaign ?
(2018 August ~)
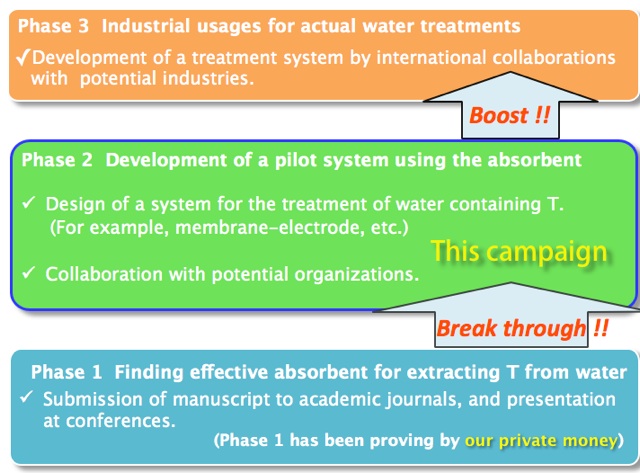
-
 Experimental results from the phase 2
Experimental results from the phase 2
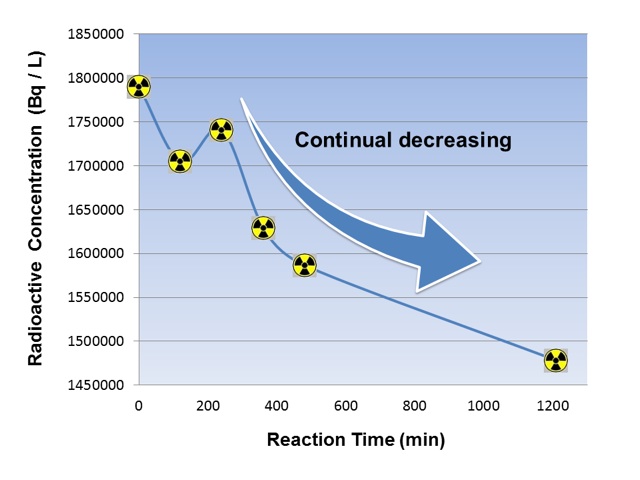
A novel extraction method using a membrane can prevent re-increasing of T concentration in the test water compared to the previous method using the powdered absorbent, published on an international academic journal (https://link.springer.com/article/10.1007/s10967-018-6022-y).
-
 This project is now (2021 April ) beginning the phase 3.
This project is now (2021 April ) beginning the phase 3.
Dependence of T-separation efficiency on initial T-activity concentration.
The latest progress in this project is explained on the Linkedin.
REFERENCES
-
1. H. Koyanaka, S. Fukutani, and H. Miyatake, Tritium separation from heavy water using a membrane containing deuterated manganese dioxide, J. Radioanalytical and Nuclear Chemistry, 322, 1889-1895, (2019) (https://doi.org/10.1007/s10967-019-06905-y)
-
2. H. Koyanaka and S. Fukutani, Tritium separation from parts-per-trillion-level water by a membrane with protonated manganese dioxide, J. Radioanalytical and Nuclear Chemistry, 318, 175-182, (2018) (https://doi.org/10.1007/s10967-018-6022-y)
-
3. H. Koyanaka and H. Miyatake, Extracting tritium from water using a protonic manganese oxide spinel, Separation Science and Technology, 50, 14, 2142-2146, (2015)